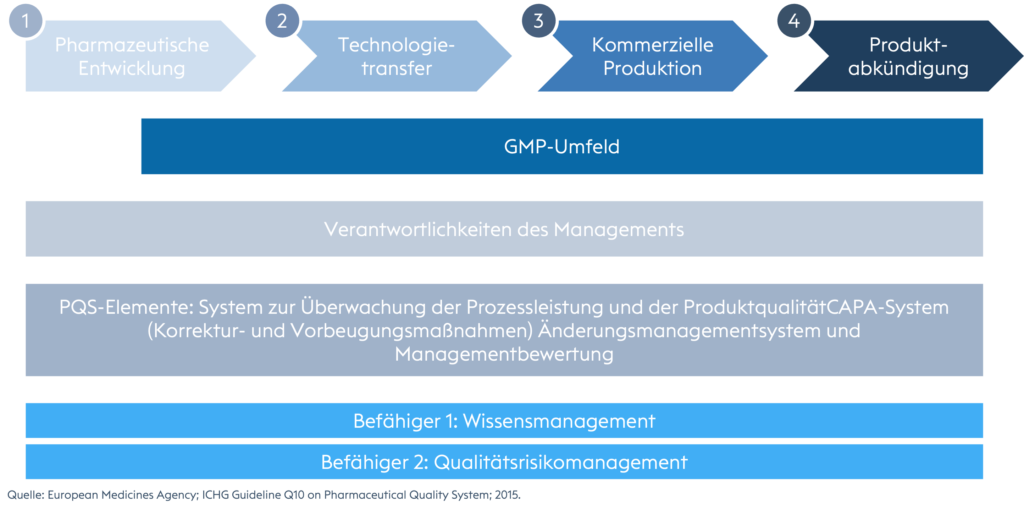
The rough process flow of pharmaceutical product development and production.
An excerpt from the current ICH Q10 Pharma Quality System.
Let's consider all the above as a kind of introduction and think about the complexity and diversity of all these steps: Collaboration across diverse interfaces and multiple departments, across international locations or even the management of third parties and service providers.
The complexity in pharma is huge and therefore one of the most regulated industries in the world.
On the one hand, and please forgive my youthful recklessness, the pharmaceutical as well as the chemical industry is quite conservative. This fact has strong advantages and disadvantages. One of the biggest advantages is that this industry focuses on true values and basic principles, which are often lost nowadays in our fast-growing, performance-oriented society, where only a minority looks right and left and considers the big picture.
Values such as respect, open-mindedness, responsiveness, punctuality and the right sense of duty usually come from managers who grew up in "good old Germany" with Helmut Kohl as chancellor.
The disadvantages are sometimes a limited willingness to change and skepticism towards new technologies.
On the other hand, once a company with such strong core values and an innovative product portfolio starts thinking about the digital revolution, a strong and competitive weapon can be formed in terms of economic profitability.
Referring to digitalization as key for there are five core pillars for improvement to overcome the lack of transparency, efficiency and limitations in the availability of consolidated data and trends, data-driven and fact-based decision making or project management, monitoring, planning and scheduling: 1.) Data collection and analysis; 2.) Process simplification and standardization; 3.) Machine performance and automation; 4.) Next generation workforce; 5.) Control tower.
The five central pillars for improvements in pharmaceutical production.
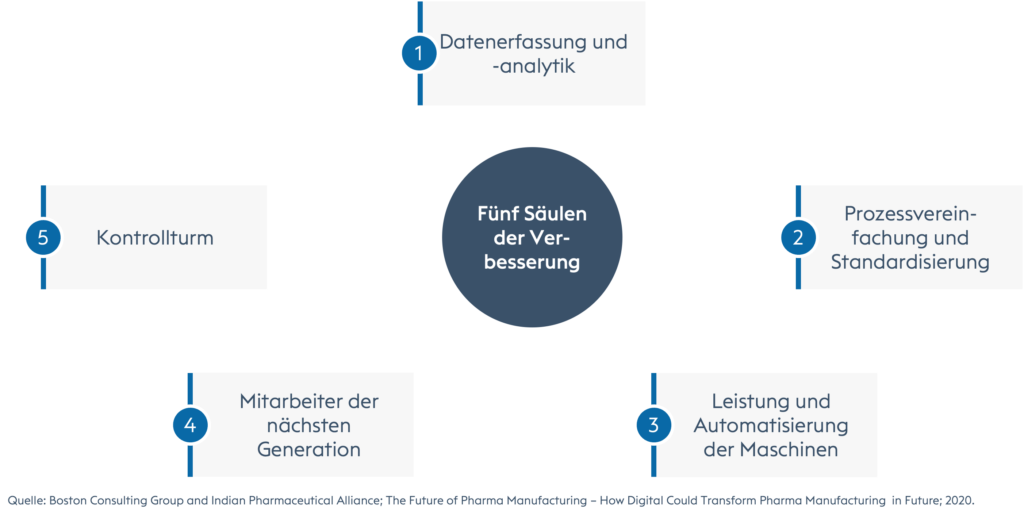
Data acquisition and advanced analytics:
In order to remain competitive, it will be important in the future - and already today - to collect data in a structured manner at various operational levels. It is then important to build advanced analytics on top of this to increase data transparency, identify opportunities for further improvement, and facilitate fact-based decision making. Companies that capture all the data, analyze it, ask the right questions and make the right decisions will set the tone for decades to come and have no competitor to fear. Take Amazon as an example.Redesign, simplification and standardization of processes:
The main driver of impact. Simplification, optimization and improvement of operational process steps through digital interventions. The results are less manual work in data search, refinement or collection, no misalignment due to a single source of truth with shared access, faster and more focused discussions and decision making due to harmonized definitions, metrics, process steps, terminology and common process understanding.Machine performance and automation:
Productivity, reliability and overall plant efficiency play a crucial role. Digital solutions are able to identify the right time windows for plant changeovers, help analyze and report out-of-spec results, facilitate well-coordinated shift handovers, support production planning, monitoring, scheduling, and flow analysis, serve as an advanced communication tool with key interfaces such as quality control, and can replace critical, quality-related process steps that are often performed manually with automation, creating a system with greater robustness, predictability, and traceability.Next Generation Employees:
A flexible workforce that is cross-trained, without bottlenecks or dependencies on any one person. Companies need to start investing more in their staff to get a well-trained and experienced workforce. It is often better to invest in existing employees who have known the company for several years rather than just focusing on new people. Of course, new talent also needs to be part of your workforce, but think about your own talent pool as well. Start an academy of sorts with change agents and train everyone else in cutting-edge technologies, new methods, tools, systems and philosophies. In the end, your company will have highly motivated, perfectly trained, and highly engaged experts ready for any challenge, expansion strategy, ups and downs, or crisis. Like the statement of a couple at the end of their marriage: for better or for worse.Control Tower:
The central nerve center of the company, or at least of the department, which is closely linked to other functions - like the airport tower in one of the world's largest metropolises. It represents the single source of truth where all data is captured, analyzed, refined, visualized and presented for decision-making or monitoring. It provides the company with real-time visibility, proactivity and better risk management with the ability to take the right corrective actions and plan preventive measures based on trends. The control tower can be equipped with artificial intelligence, Big Data analytics performed by data engineers, machine learning and automation.The 4-step journey to the "future of work" in pharmaceutical manufacturing.
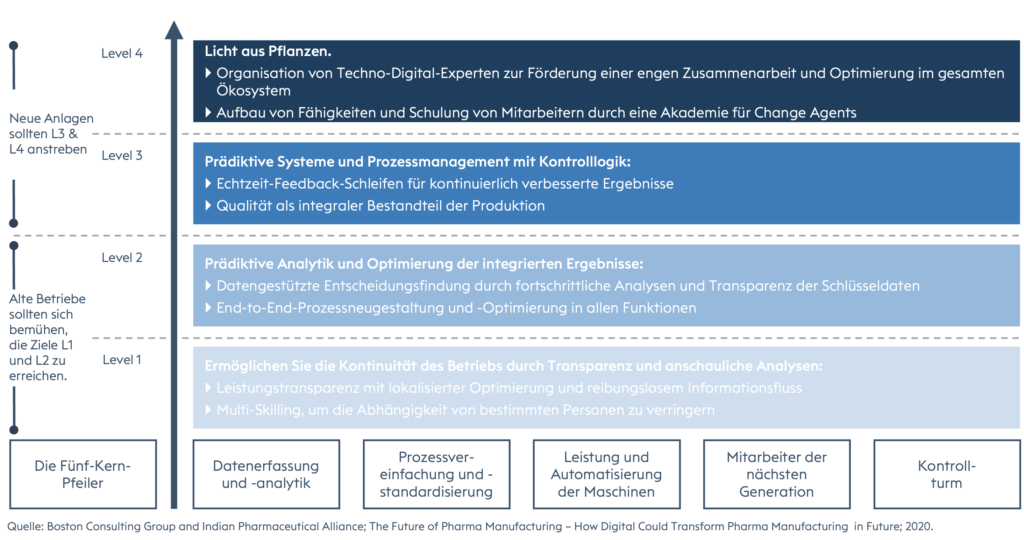
Companies embarking on such a path must first agree on a strategy with a clearly defined action plan, establish strong governance led by top management, and put in place employee-centric change management to keep employees motivated and engaged throughout such a large project and ultimately prepare them for a continuous lean and operational excellence mindset and philosophy.